Wheat rust pathogens belong to genus Puccinia, family Pucciniaceae, order Uredinales and class Basidiomycetes. These rust fungi are highly specialized plant pathogens with narrow host ranges. The Italians Fontana and Tozzetti independently provided the first unequivocal and detailed reports of wheat stem rust in 1767 (Fontana, 1932; Tozzetti, 1952). The causal organism of wheat stem rust was named P. graminis by Persoon in 1797. Chester (1946) provided one of the first detailed histories of the literature on the rust of wheat. In the early records, wheat leaf rust is not distinguished from stem rust (Chester, 1946). However, by 1815 de Candolle (1815) had shown that wheat leaf rust was caused by a distinct fungus Uredo rubigovera. The pathogen underwent a number of name changes until 1956 when Cummins and Cald-well (1956) suggested P. recondita, which has been the generally used nomenclature. Recent morphological studies by Savile (1984) and morphological and pathogen genetic studies by Anikster et al. (1997) show that P. recondita is not the incitant of wheat leaf rust. Currently P. triticina should be the preferred name as shown in by Savile (1984) and Anikster et al. (1997). This name was used by Mains and Jackson (1926) and has been used in parts of Asia and Eastern Europe for many years. In this chapter, P. triticina will be used for the leaf rust on wheat (Triticum aestivum). Although Gadd first described stripe rust of wheat in 1777, it was not until 1896 that Eriksson and Henning (1896) showed that stripe rust resulted from a separate pathogen, which they named P. glumarum. In 1953, Hylander et al. (1953) revived the name P. striiformis.
THE DISEASES
Of the rust diseases of wheat, the most common these days is called leaf or brown rust and is caused by P. triticina Eriks. It occurs on the leaf blades, although leaf sheaths can also be infected under favourable conditions, high inoculum densities, and extremely susceptible cultivars. It frequently lacks the abundant teliospore production of stem rust at the end of the season, resulting in a brown leaf lesion rather than a black stem lesion that occurs with stem rust. When leaf rust teliospores are produced, they usually emanate from telia on the lower leaf surfaces, which remain covered by the epidermal cells. The disease develops rapidly at temperatures between 10° and 30°C. Leaf rust occurs to some extent wherever wheat is grown. Losses in grain yield are primarily attributed to reduced floret set and grain shrivelling. In highly susceptible genotypes, florets, tillers and plants can be killed by early (pre-heading) epidemics. Losses due to leaf rust are usually small (less than 10 percent), but can be severe (30 percent or more). A second leaf rust occurs around the Mediterranean Sea and infects both durum and bread wheat. The pathogen is a member of the P. recondita complex (P. triticiduri V. Bourgin) and occurs in traditional agriculture systems where Anchusa italica L. serves as the primary source of inoculum (Ezzahiri et al., 1992). This pathogen produces few urediniospores, which generally occur on the lower leaf surface. Epidemics are rather local, due to the lack of urediniospores. Abundant telia are produced in a ring around the initial uredinium.
Stem rust, caused by P. graminis Pers. f. sp. tritici Eriks. & E. Henn., is also known as black rust or summer rust due to the abundant production of shiny black teliospores, which form in the uredinium at the end of the season or with unfavourable conditions. Stem rust is favoured by humid conditions and warmer temperatures of 15° to 35°C. It is the most devastating of the rust diseases and can cause losses of 50 percent in one month when conditions for its development are favourable. Losses of 100 percent can occur with susceptible cultivars.
TABLE 13.1
The rust diseases of wheat, their primary and
alternate hosts and symptoms
|
Disease |
Pathogen |
Primary hosts |
Alternate hosts |
Symptoms |
|
Leaf rust |
Puccinia triticina |
Bread and durum wheats, triticale |
Thalictrum, Anchusa, Isopyrum, Clematis |
Isolated uredinia on upper leaf surface and rarely on leaf sheaths |
|
Leaf rust duri type |
Puccinia triticiduri |
Durum and bread wheats in traditional agriculture |
Anchusa italica |
Isolated uredinia on lower leaf surface; fast teliospore development |
|
Stem rust |
Puccinia graminis f. sp. tritici |
Bread and durum wheats, barley, triticale |
Berberis vulgaris |
Isolated uredinia on upper and lower leaf surfaces, stem and spikes |
|
Stripe rust |
Puccinia striiformis f. sp. tritici |
Bread and durum wheats, triticale, a few barley cultivars |
Unknown |
Systemic uredinia on leaves and spikes and rarely on leaf sheaths |
Source: Roelfs et al., 1992.
Stripe or yellow rust, caused by P. striiformis West. f. sp. tritici Eriks. & E. Henn., is principally a disease of wheat grown in cooler climates (2° to 15°C), which are generally associated with higher elevations, northern latitudes or cooler years. It takes its name from the characteristic stripe of uredinia that produce yellow-coloured urediniospores. Because of the disease’s early attack, stunted and weakened plants often occur. Losses can be severe (50 percent) due to shrivelled grain and damaged tillers. In extreme situations, stripe rust can cause 100 percent losses.
Table 13.1 and Table 13.2 summarize primary hosts, alternate hosts, symptoms and generally accepted environmental conditions needed by the three rust diseases.
EPIDEMIOLOGY
There are several areas worldwide in which each of the rusts can cause severe losses (Saari and Prescott, 1985). In other areas, the environment is marginally suited for the diseases. Table 13.3 provides a general summary of the current and historical importance of the rust diseases worldwide.
Urediniospores of the wheat rusts initiate germination within one to three hours of contact with free moisture over a range of temperatures depending on the rust. Urediniospores are produced in large numbers and can be blown considerable distances by the wind (Hirst and Hurst, 1967; Watson and de Sousa, 1983). However, most urediniospores are deposited close to their source (Roelfs and Martell, 1984) under the influence of gravity. Urediniospores are relatively long-lived and can survive in the field away from host plants for periods of several weeks. They can withstand freezing if their moisture content is lowered to 20 to 30 percent. Viability rapidly decreases at moisture contents of more than 50 percent.
TABLE 13.2
Environmental conditions required for the wheat
rusts
|
Stage |
Temperature (°C) |
Light |
Free water | ||
|
Minimum |
Optimum |
Maximum | |||
|
Leaf rust | |||||
|
Germination |
2 |
20 |
30 |
Low |
Essential |
|
Germling |
5 |
15-20 |
30 |
Low |
Essential |
|
Appressorium |
- |
15-20 |
- |
None |
Essential |
|
Penetration |
10 |
20 |
30 |
No effect |
Essential |
|
Growth |
2 |
25 |
35 |
High |
None |
|
Sporulation |
10 |
25 |
35 |
High |
None |
|
Stem rust | |||||
|
Germination |
2 |
15-24 |
30 |
Low |
Essential |
|
Germling |
- |
20 |
- |
Low |
Essential |
|
Appressorium |
- |
16-27 |
- |
None |
Essential |
|
Penetration |
15 |
29 |
35 |
High |
Essential |
|
Growth |
5 |
30 |
40 |
High |
None |
|
Sporulation |
15 |
30 |
40 |
High |
None |
|
Stripe rust | |||||
|
Germination |
0 |
9-13 |
23 |
Low |
Essential |
|
Germling |
- |
10-15 |
- |
Low |
Essential |
|
Appressorium |
- |
- |
(not formed) |
None |
Essential |
|
Penetration |
2 |
9-13 |
23 |
Low |
Essential |
|
Growth |
3 |
12-15 |
20 |
High |
None |
|
Sporulation |
5 |
12-15 |
20 |
High |
None |
Source: Roelfs et al., 1992.
Long-distance spread of urediniospores is influenced by latitude and the respective wind patterns. In general, spores move west to east due to the winds resulting from the rotation of the earth. At progressively higher latitudes, winds tend to take a more southerly component in the Northern Hemisphere and a northerly component in the Southern Hemisphere. Studies in the United States (Roelfs, 1985a) show spore movements to be from the southwest to northeast, north of 30°N. In the Southern Hemisphere, because most of the wheat areas and land masses, in general, are north of 30°S, the movement is more west to east (Luig, 1985). How-ever, over a period of years, barley stripe rust moved south and eastward across South America (Dubin and Stubbs, 1986). In India, spores move southward probably as a result of katabatic wind flows from the mountains into the plains (Nagarajan and Joshi, 1985). In most areas studied, spores produced in the upper levels of the crop canopy move into a geographical area where the crop phenology is less advanced.
Hot days cause the air to rise from inside the canopy. When the humidity is high, fewer spores leave the uredinia. Low wind velocities dry the canopy, agitate the leaves and free the spores from the uredinia. High wind velocities may result in the release of more spores, but such winds rapidly dilute the concentration above the canopy and may be more important in generating long-distance transport than in local spread. Rain favours disease by scrubbing spores from the air, depositing them on the plants and increasing the humidity. However, rain can also wash spores from the plant surfaces, and high humidity restricts spore movement. The change in temperature due to rain will influence disease progress.
LEAF RUST
Epidemiology
Puccinia triticina can survive the same environmental conditions that the wheat leaf survives, provided infection but no sporulation has occurred. The fungus can infect with dew periods of three hours or less at temperatures of about 20°C; however, more infections occur with longer dew periods. At cooler temperatures, longer dew periods are required, for example, at 10°C a 12-hour dew period is necessary. Few if any infections occur where dew period temperatures are above 32°C (Stubbs et al., 1986) or below 2°C. Most of the severe epidemics occur when uredinia and/or latent infections survive the winter at some threshold level on the wheat crop, or where spring-sown wheat is the recipient of exogenous inoculum at an early date, usually before heading. Severe epidemics and losses can occur when the flag leaf is infected before anthesis (Chester, 1946). Puccinia triticiduri has not been intensively studied under controlled conditions but, in general, environmental conditions for infection are likely to be similar. However, the latent period (uredinial) is approximately three to four days longer, and teliospore production starts shortly after initial urediniospore production. The initial inoculum (aeciospores) is from A. italica, and the disease spread is generally limited.
TABLE 13.3
Current and historical importance of wheat
leaf, stem and stripe rusts for the epidemiological zones of Saari and
Prescott
|
Zone |
Leaf rust |
Stem rust |
Stripe rust | |||
|
Currenta |
Historical |
Current |
Historical |
Current |
Historical | |
|
Africa | ||||||
|
North |
Major |
Major |
Local |
Major |
Local |
Local |
|
East |
Local |
Local |
Major |
Major |
Major |
Major |
|
Southern |
Local |
Local |
Local |
Major |
Local |
Rare |
|
Asia | ||||||
|
Far East |
Local |
Local |
Local |
Major |
Major |
Major |
|
Central |
Major |
Major |
Minor |
Minor |
Local |
Local |
|
South |
Local |
Major |
Minor |
Major |
Local |
Local |
|
Southeast |
Major |
Major |
Minor |
Minor |
Rare |
Rare |
|
West |
Local |
Local |
Local |
Major |
Major |
Major |
|
Australia, |
Local |
Local |
Local |
Major |
Local |
Rare |
|
Europe | ||||||
|
East |
Major |
Major |
Minor |
Major |
Local |
Local |
|
West |
Local |
Major |
Minor |
Major |
Major |
Major |
|
North America |
Major |
Major |
Minor |
Major |
Local |
Local |
|
South America |
Major |
Major |
Local |
Major |
Local |
Local |
aMajor = severe losses without the cultivation of resistant varieties; Minor = usually occurs, but of little significance; Local = only occurs in a small part of the region, losses in these areas may be occasionally severe if susceptible cultivars are grown; Rare = not present, rarely seen, or as in Australia and New Zealand, recently introduced.
Source: Saari and Prescott, 1985
Hosts
The P. recondita complex members attack a wide number of grasses; yet there seems to be a strict specialization of the host range of the different populations. Puccinia triticina is primarily a pathogen of wheat, its immediate ancestors and the man-made crop triticale. The P. recondita f. sp. secalis from rye does not attack wheat. Recent evidence (Huerta-Espino and Roelfs, 1989) indicates that populations of leaf rust exist in Europe, Asia and Africa that are primarily pathogens of durum wheat. They are all distinct from the population that exists worldwide on bread wheat.
Alternate hosts
The fungus produces its sexual gametes (pycniospores and receptive hyphae) on the alternate host. Most rust researchers assume that Thalictrum speciosissimum (in the Ranunculaceae family) is the primary alternate host for P. recondita f. sp. tritici in Europe. Alternate hosts seldom, if ever, function in North America (Saari et al., 1968), South America and Australia. Clematis spp. and Isopyrum were reported to be infected by P. recondita (Table 13.1). The alternate host is considered important at least for recombining virulence factors in part of the Mediterranean area (d’Oliveira and Samborski, 1966). The importance of the alternate host in generating changes in the pathogen population for virulence combinations and other factors worldwide is unknown.
Recent studies (Anikster et al., 1997) indicated that the primary alternate host of P. triticina, including the durum attacking populations, is T. speciosissimum. Whereas A. agregata, A. undulata, Echium glomeratum and Lycopsis arvensis (Boraginaceae) were the alternate host for the leaf rust on wild wheats (Triticum [Aegilops] spp.) and rye. However, in the Mediterranean area, a second leaf rust P. triticiduri on bread and durum wheat has basidiospores that attack A. italica (Ezzahiri et al., 1992; Anikster et al., 1997). The alternate host is essential for the survival and spread of P. triticiduri.
The alternate host is infected when the teliospores germinate in the presence of free moisture. Basidiospores (1n) are produced that are capable of being carried a short distance (a few metres) to infect the alternate hosts. Approximately seven to ten days following infection, pycnia with pycniospores and receptive hyphae appear. These serve as the gametes, and fertilization occurs when the nectar containing the pycniospores is carried to receptive hyphae of the other mating type by insects, by splashing rain, or by cohesion. The aecial cups appear seven to ten days later on the lower surface of the leaf, producing aeciospores that are windborne and that cause infection by penetrating the stomata of the wheat leaves (Figure 13.1). The distances travelled by aeciospores appear to be relatively short.
Accessory hosts
The members of the P. recondita complex attack many species of grasses, but it is unclear which species serve as functional hosts in nature. Many grasses can be infected by artificial inoculation, however, this may not occur in the field. Potential hosts for wheat leaf rusts could be wild or weedy species of the genera Triticum and Aegilops (now classified as Triticum) and the related species of the Agropyron complex and Secale. Agropyron repens L. has been reported as a host for P. recondita (P. persistent sp. persistens f. agropyrina [Eriks.] Urban et Markova), which easily transfers from and to wheat (Azbukina, 1980). In southern Italy, Agropyron sp. is also reported to be infected by a wheat- and Thalictrum-infecting rust (Casulli, 1988). The most common non-crop host for wheat leaf rust is volunteer or self-sown wheat. These plants may be in fallow fields, along the edges of fields and roads, as weeds in a second crop and as a cover crop under orchards, along irrigation canals, etc. This is the major source of inoculum throughout much of the world where wheat is autumn- or winter-sown. Currently no accessory hosts are known for P. triticiduri.
FIGURE 13.1
Life and disease cycles for
Puccinia tricina
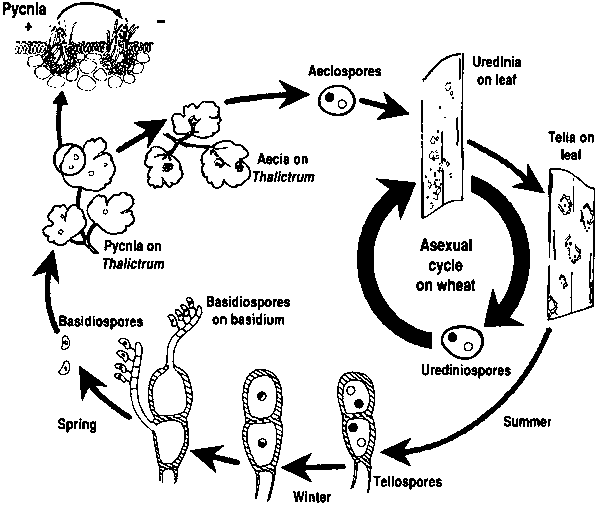
Source: Courtesy V.Brewster.
Primary hosts
The primary host of P. triticina is T. aestivum L. em. Thell. It has generally been of lesser importance on T. turgidum L., except in the Mediterranean, the Middle East, Ethiopia, Chile and India, where durum wheats are more extensively cultivated. It is of minor importance on T. monococcum L., T. dicoccum and Ae. speltoides Tausch. Wheat leaf rust would also appear to be a major threat to triticale (X Triticosecale Wittmack), the crop derived from the man-made cross between wheat and rye (Skovmand et al., 1984). Puccinia triticiduri is a pathogen of T. turgidum and some T. aestivum cultivars. This leaf rust is limited to regions around the Mediterranean Sea where A. italica is common and where traditional methods of cultivation allow the perennial Anchusa to survive in wheat fields.
Pathogen
De Candolle (1815) separated wheat leaf rust from other rusts of wheat and called it U. rubigovera in 1815. Eriksson and Henning (1894) described the causal organisms of both wheat and rye leaf rust as P. dispersa. Eriksson (1894) separated the wheat and rye leaf rust fungi, and the causal organism of wheat leaf rust became P. triticina, a name still used in parts of Eastern Europe. Mains (1932) placed the causal organism of wheat leaf rust in P. rubigovera and established a complex group of 52 formae speciales for the fungus causing wheat leaf rust. Puccinia recondita was recommended by Cummins and Caldwell (1956) as the correct designation for the wheat leaf rust pathogen. Puccinia recondita f. sp. tritici was widely used thereafter (Samborski, 1985). However, in 1984, Savile (1984) stated that P. triticina was the binomial for wheat leaf rust and P. recondita for rye leaf rust. Recently, an extensive study (Anikster et al., 1997) of morphologic characters, alternate host ranges and the intermating of cultures within and among various populations showed that P. triticina and P. recondita are different organisms. Puccinia recondita f. sp. secalis and P. triticiduri are more similar and share some ability to attack the same alternate host species.
Life cycle
Figure 13.1 shows the life cycle for P. triticina and P. triticiduri and the disease cycle for wheat leaf rust. The time for each event and frequency of some events (sexual cycle, wheat cropping season and green-bridge) may vary among areas and regions of the world.
The alternate host currently provides little direct inoculum of P. triticina to wheat, but may be a mechanism for genetic exchanges between races and perhaps populations. The pathogen survives the period between wheat crops in many areas on a green-bridge of volunteer (self-sown) wheat (see section "Epidemiology"). Inoculum in the form of urediniospores can be blown by winds from one region to another. The sexual cycle is essential for P. triticiduri. Teliospores can germinate shortly after development, and basidiospore infection can occur throughout the wheat-growing cycle.
Urediniospores initiate germination 30 minutes after contact with free water at temperatures of 15° to 25°C. The germ tube grows along the leaf surface until it reaches a stoma; an appressorium is then formed, followed immediately by the development of a penetration peg and a sub-stomatal vesicle from which primary hyphae develop. A haustorial mother cell develops against the mesophyll cell, and direct penetration occurs. The haustorium is formed inside the living host cell in a compatible host-pathogen interaction. Secondary hyphae develop resulting in additional haustorial mother cells and haustoria. In an incompatible host-pathogen response, haustoria fail to develop or develop at a slower rate. When the host cell dies, the fungus haustorium dies. Depending upon when or how many cells are involved, the host-pathogen interaction will result in a visible resistance response (Rowell, 1981, 1982).
Spore germination to sporulation can occur within a seven- to ten-day period at optimum and constant temperatures. At low temperatures (10° to15°C) or diurnal fluctuations, longer periods are necessary. The fungus may survive as insipid mycelia for a month or more when temperatures are near or below freezing. Maximum sporulation is reached about four days following initial sporulation (at about 20°C). Although the number can vary greatly, about 3 000 spores are produced per uredinium per day. This level of production may continue for three weeks or more if the wheat leaf remains alive that long (Chester, 1946; Stubbs et al., 1986). Uredinia (pustules) are red, oval-shaped and scattered, and they break through the epidermis (Plate 12). Urediniospores are orangered to dark red, echinulate, spherical and usually measure 20 to 28 µm in diameter (Plate 13). The teliospores (Plate 14) are dark brown, two-celled with thick walls and rounded or flattened at the apex (Plate 15). Puccinia triticiduri differs from P. triticina in requiring 10 to 12 days for appearance of urediniospores, and initial teliospore production often occurs within 14 days of the initial infection. The uredinia are yellowish-brown and produce many fewer urediniospores per uredinia, and within a few days the lesion primarily produces teliospores. Also P. triticiduri infections are likely to be on the lower leaf surface.
The teliospores of P. triticina are formed under the epidermis with unfavourable conditions or senescence and remain with the leaves. Leaf tissues can be dispersed or moved by wind, animals or humans to considerable distances. Basidiospores are formed and released under humid conditions, which limit their spread. Basidiospores are also hyaline and sensitive to light, further limiting travel to probably tens of metres. Aeciospores are more similar to urediniospores in their ability to be transported by wind currents, but long-distance transport has not been noted for some reason. Puccinia triticiduri will produce abundant teliospores within weeks of the initial infection, producing a dark ring telia around each infection site.
Virulence
Virulence, or the ability of a pathogen to overcome a specific gene for resistance, probably exists for almost all numbered major Lr genes on a worldwide basis. Because virulence exists for most of the resistance genes singly and for various combinations of two or more genes, it is essential to know what combination of virulence exists in the pathogen population before spending time on combining resistances in a host cultivar. This requires a systematic pathogen survey from which samples are obtained from different cultivars and different geographical and ecological areas throughout the season. In most areas, the rust (thus virulences) can survive the entire year in the asexual cycle. To the authors’ knowledge, P. triticiduri does not survive more than a single season in the uredinial stage. Genetic recombinations of virulence can occur several times in a single crop season (Ezzahiri et al., 1992).
STEM RUST
Stem or black rust of wheat is caused by P. graminis f. sp. tritici. At one time, it was a feared disease in most wheat regions of the world. The fear of stem rust was under-standable because an apparently healthy crop three weeks before harvest could be reduced to a black tangle of broken stems and shrivelled grain by harvest. In Europe and North America, the removal of the alternate host reduced the number of combinations of virulence and the amount of locally produced inoculum (aeciospores). In addition, in some areas early maturing cultivars were introduced to permit a second crop or to avoid flowering and grain-filling during hot weather. Early maturing cultivars escape much of the damage caused by stem rust by avoiding the growth period of the fungus. The widespread use of resistant cultivars worldwide has reduced the disease as a significant factor in production. Although changes in pathogen virulence have rendered some resistances ineffective, resistant cultivars have generally been developed ahead of the pathogen. The spectacular epidemics that developed on Eureka (Sr6 in Australia) in the 1940s and on Lee (Sr9g, Sr11, Sr16), Langdon (Sr9e, +) and Yuma (Sr9e, +) in the United States in the mid-1950s really have been the exceptions in the past. The experience in other parts of the world has been similar (Luig and Watson, 1972; Roelfs, 1986; Saari and Prescott, 1985). Today, stem rust is largely under control worldwide (Table 13.3).
Epidemiology
The epidemiology of P. graminis is similar to P. triticina. The minimum, optimum and maximum temperatures for spore germination are 2°, 15° to 24°, and 30°C, respectively (Hogg et al., 1969) and for sporulation, 5°, 30° and 40°C, respectively, which is about 5.5°C higher in each category than for P. triticina. Stem rust is more important late in the growing period, on late-sown and maturing wheat cultivars, and at lower altitudes. Spring-sown wheat is particularly vulnerable in the higher latitudes if sources of inoculum are located downwind. Large areas of autumn-sown wheat occur in the southern Great Plains of North America, providing inoculum for the northern spring-sown wheat crop. In warm humid climates, stem rust can be especially severe due to the long period of favourable conditions for disease development when a local inoculum source is available.
Stem rust differs from leaf rust in requiring a longer dew period (six to eight hours are necessary). In addition, many penetration pegs fail to develop from the appressorium unless stimulated by at least 10 000 lux of light for a three-hour period while the plant slowly dries after the dew period. Maximum infection is obtained with 8 to 12 hours of dew at 18°C followed by 10 000+ lux of light while the dew slowly dries and the temperature rises to 30°C (Rowell, 1984). Light is seldom limiting in the field as dews often occur in the morning. However, little infection results when evening dews and/or rains are followed by winds causing a dry-off prior to sunrise. In the greenhouse, reduced light is often the reason for poor infection rates. The effect of light probably is an effect on the plant rather than the fungus system as urediniospores injected inside the leaf whorl result in successful fungal penetrations without light striking the fungus. Stem rust uredinia occur on both leaf and stem surfaces as well as on the leaf sheaths, spikes, glumes, awns and even grains.
A stem rust pustule (uredinium) can produce 10 000 urediniospores per day (Katsuya and Green, 1967; Mont, 1970). This is more than leaf rust, but the infectability is lower with only about one germling in ten resulting in a successful infection. Stem rust uredinia, being mostly on stem and leaf sheath tissues, often survive longer than those of leaf rust, which are confined more often to the leaf blades. The rate of disease increase for the two diseases is very similar.
Stem rust urediniospores are rather resistant to atmospheric conditions if their moisture content is moderate (20 to 30 percent). Long-distance transport occurs annually (800 km) across the North American Great Plains (Roelfs, 1985a), nearly annually (2000 km) from Australia to New Zealand (Luig, 1985) and at least three times in the past 75 years (8 000 km) from East Africa to Australia (Watson and de Sousa, 1983).
Aeciospores can also be a source of inoculum of wheat stem rust. Historically, this was important in North America and northern and eastern Europe. This source of inoculum has generally been eliminated or greatly reduced by removal of the common or European barberry (Berberis vulgaris) from the proximity of wheat fields. Aeciospores infect wheat similarly to urediniospores.
Hosts
Wheat, barley, triticale and a few related species are the primary hosts for P. graminis f. sp. tritici. However, the closely related pathogen, P. graminis f. sp. secalis, is virulent on most barleys and some wheats (e.g. Line E). Puccinia graminis f. sp. secalis can attack Sr6 and Sr11 in a Line E host background (Luig, 1985). The primary alternate host in nature has been B. vulgaris L., a species native to Europe, although other species have been susceptible in greenhouse tests. The alternate hosts are usually susceptible to all or none of the formae speciales of P. graminis.
Alternate hosts
The main alternate host for P. graminis is B. vulgaris, which was spread by humans across the northern latitudes of the Northern Hemisphere. Because of its upright, bushy growth with many sharp thorns, it made an excellent hedge along field borders. The wood was useful for making tool handles, the bark provided a dye and the fruit was used for making jams. Settlers coming to North America from Europe brought the barberry with them. The barberry spread westward with humans and became established as a naturalized plant from Pennsylvania through the eastern Dakotas and southward into north-eastern Kansas. Many species of Berberis, Mahonia and Mahoberberis are susceptible to P. graminis (Roelfs, 1985b). The Canadian or Allegheny barberry, B. canadensis, should be added to this list.
The alternate host was a major source of new combinations of genes for virulence and aggressiveness in the pathogen (Groth and Roelfs, 1982). The amount of variation in the pathogen made breeding for resistance difficult, if not impossible. Of the virulence combinations present one year, many would not reoccur the following year, but many new ones would appear (Roelfs, 1982). The barberry was the source of inoculum (aeciospores) early in the season. Generally, infected bushes were close to cereal fields of the previous season, so inoculum travelled short distances without the loss in numbers and viability associated with long-distance transport. A single large barberry bush can produce about 64 x 109 aeciospores in a few weeks (Stakman, 1923). This is the equivalent of the daily output of 20 million uredinia, in an area of 400 m2.
Barberry was a major source of stem rust inoculum in Denmark (Hermansen, 1968) and North America (Roelfs, 1982). The success of reducing stem rust epidemics in northern Europe and North America following the removal of barberry near wheat fields has probably led to an overemphasis of the role of this alternate host in generating annual epidemics elsewhere.
Resistance to P. graminis in Berberis is reported to result from the inability of the pathogen to directly penetrate the tough cuticle (Melander and Craigie, 1927). Berberis vulgaris becomes resistant to infection about 14 days after the leaves unfold. However, infections occur on the berries, thorns and stems, which suggests the toughening of the cuticle may not be as important as originally thought. In recent testing of alternate host cultivars, a hypersensitive response has been observed particularly with Berberis spp. (Mahonia).
Accessory hosts
It is necessary to separate the accessory hosts for P. graminis f. sp. tritici from those of the other formae speciales, especially those of P. graminis f. sp. secalis. Additionally, many other grasses can be infected as seedlings in the greenhouse or as adult plants when spores are directly injected into the leaf whorl, but are rust-free under field conditions.
Barley, triticale and an occasional rye plant are infected by wheat stem rust. Wild Hordeum spp., such as H. jubatum L. and rarely H. pusillum Nutt., and T. (Ae.) cylindrica Host are sometimes infected in the United States (Roelfs, 1986); however, it is thought that the inoculum generally comes from wheat to these grasses rather than vice versa in North America.
Primary hosts
Triticum aestivum L. and T. turgidum L. as cultivated wheats and triticale are the primary hosts of wheat stem rust.
Pathogen
Fontana (1932) made the first known detailed study, including precise drawings, of P. graminis in 1767. Persoon named the fungus on barberry Aecidium berberidis in 1791 and the form on wheat P. graminis in 1794. DeBary (1866) showed that the two fungi were different stages of a single species. Craigie (1927) made the first controlled crosses between strains of P. graminis.
FIGURE 13.2
Life and disease cycles for Puccinia
graminis
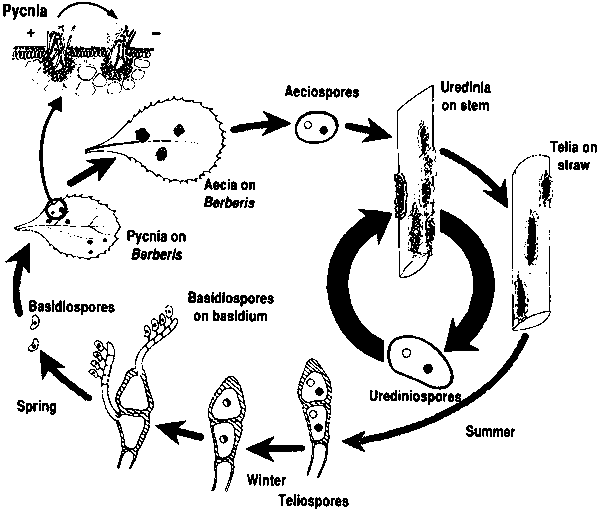
Souce: Courtesy of V. Brewater.
Life cycle
In most areas of the world, the life cycle (Figure 13.2) of P. graminis f. sp. tritici consists of continual uredinial generations. The fungus spreads by airborne urediniospores from one wheat plant to another and from field to field. Primary inoculum may originate locally (endemic) from volunteer plants or be carried long distances (exodemic) by wind and deposited by rain. In North America, P. graminis annually moves 2 000 km from the southern winter wheats to the most northern spring wheats in 90 days or less and in the uredinial cycle can survive the winter at sea level to at least 35°N. Snow can provide cover that occasionally permits P. graminis to survive as infections on winter wheat even at severe sub-freezing temperatures experienced at 45°N (Roelfs and Long, 1987). The sexual cycle seldom occurs except in the Pacific Northwest of the United States (Roelfs and Groth, 1980) and in local areas of Europe (Spehar, 1975; Zadoks and Bouwman, 1985). Although the sexual cycle produces a great genetic diversity (Roelfs and Groth, 1980), it also produces a large number of individuals that are less fit due to frequent recessive virulence genes (Roelfs and Groth, 1988) and to reassortment of genes for aggressiveness. Puccinia graminis has successfully developed an asexual reproduction strategy that apparently allows the fungus to maintain necessary genes in blocks that are occasionally modified by mutation and selection.
Urediniospore germination starts in one to three hours at optimum temperatures (Table 13.2) in the presence of free water. The moisture or dew period must last six to eight hours at favourable temperatures for the spores to germinate and produce a germ tube and an appressorium. Visible development will stop at the appressorium stage until at least 10 000 lux (16 000 being optimum) of light are provided. Light stimulates the formation of a penetration peg that enters a closed stoma. If the germling dries out during the germination period, the process is irreversibly stopped. The penetration process takes about three hours as the temperature rises from 18° to 30°C (Rowell, 1984). The light requirement for infection makes P. graminis much more difficult to work with in the greenhouse than P. recondita. Most likely, light seldom has an effect in the field except when dew periods dissipate before daybreak.
Urediniospores develop in pustules (uredinia) that rupture the epidermis and expose masses of reddish-brown spores. The uredinia are larger than those of leaf rust and are oval-shaped or elongated, with loose or torn epidermal tissue along the margins (Plate 16). The urediniospores are reddish-brown, elliptical to egg-shaped, echinulate structures measuring 24 to 32 µm x 18 to 22 µm (Plate 17).
As the host matures, telia (Plate 18) are produced directly from urediniospore infections or teliospores can be produced in a mature uredinial pustule. The teliospores are dark brown two-celled and somewhat wedge-shaped. They have thick walls, and measure 40 to 60 µm x 18 to 26 µm. The apical cell is rounded or slightly pointed (Plate 19). The teliospores are dicaryotic (n + n) and remain with the straw until spring. During this time, karyogamy occurs and the teliospores become diploid (2n). With spring rains and favourable temperatures, the teliospore germinates, un-dergoes meiosis and produces a four-celled basidium. Each cell produces a stigma with a single haploid basidiospore (1n). The hyaline basidiospore is windborne short distances (metres) to the barberry bush. Basidiospores germinate and penetrate directly. For maximum infection, the barberry leaf tissue should be less than two weeks old. Infection by a basidiospore results in the production of a pycnium (1n). The pycnium produces receptive hyphae and pycniospores of a single mating type (+ or -) that serve as female and male gametes for the fungus. Pycniospores of one mating type must be transferred to the receptive hyphae of the opposite mating type to initiate aeciospore development. The transfer of pycniospores is frequently done by insects, which are attracted to the oozing pycnial nectar produced by the pycnium. Mating of + and - types can also be facilitated by splashing rain, brushing of leaves, larger animals and neighbouring infections that coalesce. Aeciospores are dicaryotic (n + n) and are produced in aecia generally on the lower surface of the barberry leaves seven to ten days following fertilization. The aeciospores are the products of genetic recombination and may differ in their virulence and aggressiveness. The extent of variation depends on the differences between the parental isolates. Puccinia graminis f. sp. tritici has been crossed with other formae speciales, and crosses with P. graminis f. sp. secalis were relatively fertile (Johnson, 1949). In Australia, evidence points to recombination of wheat stem rust and the scabrum rust (P. graminis f. sp. secalis) (Burdon et al., 1981; Luig and Watson, 1972).
Aeciospores are hydroscopically released from the aecia and are airborne to wheat over distances of metres to perhaps a few kilometres. Aeciospores require similar conditions for infection to those of urediniospores. Infection by aeciospores results in the production of dicaryotic (n + n) uredinia with urediniospores. The repeating asexual cycle then involves urediniospores producing uredinia in about a 14-day cycle with optimum conditions. Under field conditions, where temperatures vary greatly, the cycle can be either lengthened or shortened. Generally, lower temperatures in the field, at least in the early stages of the crop cycle, tend to lengthen the latent period. In northern India, a latent period of 31 days was recorded for stem rust (Joshi and Palmer, 1973).
Virulence
Worldwide virulence for resistance genes Sr2, 13, 22, 24, 25, 26, 27, 29, 31, 32, 33, 34, 37, Gt and Wld-1 is limited or undetected. Sr13 is ineffective at low temperatures, 18° to 20°C. Sr29 and 34 may be ineffective under high inoculum densities. Virulence for Sr24 exists in South Africa (Le Roux and Rijkenberg, 1987) and Madagascar, for Sr25 in India and for Sr27 in Australia (McIntosh et al., 1983). Isolates often appear to be virulent on Sr24, 29, 34, Gt and Wld in the field due to the low level of effectiveness under high inoculum densities. Sr37 virulent isolates have so far been unconfirmed and perhaps were obtained from the off-type plants. Virulence for Sr26 has been undetected despite the widespread use of the cultivar Eagle and its derivatives in Australia. Likewise, the wide use of Sr31 in Kavkaz and similar wheats with the 1BL.1RS translocation did not result in virulence for Sr31 until 1999 when virulence was detected in Uganda (Pretorius et al., 2000).
STRIPE RUST
Stripe or yellow rust of wheat caused by P. striiformis f. sp. tritici can be as damaging as stem rust. However, stripe rust has a lower optimum temperature for development that limits it as a major disease in many areas of the world. Stripe rust is principally an important disease of wheat during the winter or early spring or at high elevations. Table 13.3 shows regions of the world where stripe rust has been a major or local problem.
Stripe rust of wheat may be the cause of stripe rust on barley (Stubbs, 1985). In Europe, a forma specialis of P. striiformis has evolved that is commonly found on barley and seldom on any but the most susceptible wheats (Zadoks, 1961). Puccinia striiformis f. sp. hordei was introduced into South America where it spread across the continent (Dubin and Stubbs, 1986) and was later identified in Mexico and United States (Roelfs et al., 1992).
Epidemiology
Puccinia striiformis has the lowest temperature requirements of the three wheat rust pathogens. Minimum, optimum and maximum temperatures for stripe rust infection are 0°, 11° and 23°C, respectively (Hogg et al., 1969). Puccinia striiformis frequently can actively overwinter on autumn-sown wheat. Most of the epidemiology work has been done in Europe and recently reviewed by Zadoks and Bouwman (1985) and Rapilly (1979).
In Europe, P. striiformis oversummers on wheat (Zadoks, 1961). The amount of over-summering rust depends on the amount of volunteer wheat, which, in turn, is a function of moisture in the off-season. The ured-iniospores are then blown to autumn-sown wheat. In northwestern Europe, overwintering is limited to urediniomycelia in living leaf tissues as temperatures of -4°C will kill exposed sporulating lesions. Latent lesions can survive if the leaf survives. In other areas of the world, snow can insulate the sporulating lesions from the cold temperatures so air temperatures below -4°C fail to eliminate the rust lesions. The latent period for stripe rust during the winter can be up to 118 days and is suspected to be as many as 150 days under a snow cover (Zadoks, 1961).
In areas near the equator, stripe rust tends to cycle endemically from lower to higher altitudes and return following the crop phenology (Saari and Prescott, 1985). In more northern latitudes, the cycle becomes longer in distance with stripe rust moving from mountain areas to the foothills and plains.
Due to their susceptibility to ultraviolet light, urediniospores of stripe rust probably are not transported in a viable state as far as those of leaf and stem rusts. Maddison and Manners (1972) found stripe rust urediniospores three times more sensitive to ultraviolet light than those of stem rust. Still, Zadoks (1961) reports stripe rust was wind-transported in a viable state more than 800 km. The introductions of wheat stripe rust into Australia and South Africa and barley stripe rust into Colombia were probably aided by humans through jet travel (Dubin and Stubbs, 1986; O’Brien et al., 1980). However, the spread of stripe rust from Australia to New Zealand, a distance of 2 000 km, was probably through airborne urediniospores (Beresford, 1982). Perhaps an average spore of stripe rust has a lower likelihood of being airborne in a viable state over long distances than that of the other wheat rusts, but certainly some spores must be able to survive long-distance transport under special and favourable conditions. There are several examples of the sequential migration of stripe rust. Virulence for gene Yr2 (cultivars Siete Cerros, Kalyansona and Mexipak) was first recorded in Turkey and over a period of time was traced to the subcontinent of India and Pakistan (Saari and Prescott, 1985) and may be associated with the weather systems called the ‘Western Disturbance’. As mentioned, barley stripe rust in South America migrated from its introduction point in Colombia to Chile over a period of a few years (Dubin and Stubbs, 1986).
Most areas of the world studied seem to have a local or nearby source of inoculum from volunteer wheat (Line, 1976; Stubbs, 1985; Zadoks and Bouwman, 1985). However, some evidence points to inoculum coming from non-cereal grasses (Hendrix et al., 1965; Tollenaar and Houston, 1967). Future studies of stripe rust epidemiology need to take into account not only the presence of rust on nearby grasses, but also the fact that the rust must occur on the grasses prior to its appearance on cereals. The virulence phenotype must be shown to be the same on both hosts and that it moves from the grass to wheat during the crop season.
Stripe rust epidemics in the Netherlands can be generated by just a single uredinium per hectare surviving the winter if the spring season is favourable for rust development (Zadoks and Bouwman, 1985). Visual detection of a single uredinium per hectare is unlikely, however, as foci develop around the initial uredinium, it becomes progressively easier to detect.
Hosts
Puccinia striiformis is a pathogen of grasses and cereal crops: wheat, barley, triticale and rye. Stripe rust is the only rust of wheat that consistently spreads beyond the initial infection point within the plant.
Alternate hosts
Only the telial and uredinial stages of stripe rust are known. Eriksson and Henning (1894) looked for the alternate host among species of the Boraginaceae. Tranzschel (1934) suggested that Aecidium valerianella, a rust of valerianella, might be related to P. striiformis. Mains (1933) thought that P. koeleriae Arth., P. arrhenatheri Eriks. and P. montanensis Ellis, which have aecidial states on Berberis and Mahonia spp., might be related to P. striiformis.
Straib (1937) and Hart and Becker (1939) were unsuccessful in attempts to infect Berberis, Mahonia and Valerianella spp. The alternate host of the rust, P. agropyri Ell. & Ev., is Clematis vitalba. This rust closely resembles P. striiformis thus Viennot-Bourgin (1934) suggested that the alternate host of stripe rust might occur in the Clematis family. Teliospores readily germinate immediately to produce basidiospores (Wright and Lennard, 1980), and the teliospores probably do not assist the fungus as a winter survival mechanism. An epidemiological factor to consider is the possibility of infection of the alternate host late in the summer so aeciospores could infect the newly sown wheat or late cool season grasses. In some high-altitude areas of West Asia, the wheat crop may take 13 months to mature. In such cases, early spring season infections of the alternate host would be possible.
Accessory hosts
Puccinia striiformis seems to lack the clearly defined formae speciales that occur with P. graminis, and isolates of stripe rust seem to have a wider host range than those of P. recondita. Sufficient evidence exists for the separation of the primary wheat attacking form from the barley attacking form (Stubbs, 1985; Zadoks, 1961).
Puccinia striiformis attacks members of the subfamilies Festucoideae and Eragrostoideae with the principal hosts in the genera Aegilops (Triticum), Agropyron, Bromus, Elymus, Hordeum, Secale and of course Triticum (Stubbs, 1985). The assumption that stripe rust, which occurs on various grass species, has a similar virulence to that which attacks wheat is probably not justified (Manners, 1960; Tollenaar and Houston, 1967). Likewise, the ability to produce a few uredinia on some plants of a species in greenhouse tests does not prove that the species is a host under field conditions. Furthermore, there is no reason to expect that race-specific resistance does not occur in accessory hosts. Many of the existing race-specific genes for resistance have been transferred from species that are accessory hosts.
Primary hosts
Triticum spp. are a major host for stripe rust. Stripe rust on barley in Tibet has historically been an important disease where wheat is a minor crop. Comparisons between Tibetan and European stripe rust remain to be carried out. Rye was often reported as a host of stripe rust in the last century, but in more recent times, rye is seldom seen to be infected by stripe rust (Stubbs, 1985).
Pathogen
Gadd and Bjerkander first described stripe rust in 1777. It was reported to have caused an epidemic on rye in Sweden in 1794 (Eriksson and Henning, 1896). Schmidt designated the pathogen as U. glumarum in 1827; Westendorp designated the stripe rust pathogen of rye as P. sfriaeformis in 1854. Eriksson and Henning (1896) chose the name P. glumarum in their comprehensive taxonomic work. Hylander et al. (1953) and Cummins and Stevenson (1956) revived the name currently in use, P. striiformis West. It probably is desirable to add the forma specialis if it has been determined.
Life cycle
Puccinia striiformis is most likely a hemiform rust in that the life cycle seems only to consist of the uredinial and telial stages. Uredia develop in narrow, yellow, linear stripes mainly on leaves and spikelets (Plate 20). When the heads are infected, the pustules appear on the inner surfaces of glumes and lemmas (Plate 21). The urediniospores are yellow to orange in colour, more or less spherical, echinulate and 28 to 34 µm in diameter (Plate 22). Narrow black stripes are formed on leaves during telial development. Teliospores are dark brown, two-celled and similar in size and shape to those of P. triticina (Plate 23). Stripe rust populations can exist, change in virulence and result in epidemics independent of an alternate host. Urediniospores are the only known source of inoculum for wheat, and they germinate and infect at cooler temperatures.
Virulence
On a worldwide basis, virulence probably exists for most numbered Yr genes. However, virulence on certain gene combinations may be absent on a regional basis. Virulence on the adult genes is unknown, as most virulence surveys are done only in the seedling stage.
DISEASE CONTROL
It cannot be overemphasized that it is essential to understand the epidemiology of a disease before starting any control strategy, especially one involving cultural or chemical control measures. Without doubt, a combination of cultural control practices with disease resistance and perhaps fungicide applications (under unusual circumstances) will be the most effective means of controlling the cereal rust diseases. Because of the airborne nature of the inoculum of cereal rusts, quarantine measures against the pathogen only delay, and do not prevent, entry of the disease and/or specific virulence combinations. However, one should take care not to unknowingly transport or permit urediniospores of the cereal rusts to escape outside their epidemiological areas. Important differences in virulence, aggressiveness and adaptation exist in the different pathogen populations of these fungi worldwide.
Genetic resistance
The principle mechanism of control of the cereal rusts has been through the use of resistant cultivars (Johnson, 1981). A few historical cultivars, such as Thatcher and Hope (Hare and McIntosh, 1979) for stem rust, Americano 25, Americano 44d, Surpreza, Frontana and Fronteira (Perez and Roelfs, 1989; Roelfs, 1988) for leaf rust, and Wilhelmina, Capelle-Desprez, Manella, Juliana and Carstens VI (Stubbs, 1985) for stripe rust, have maintained some resistance for many years. Most cultivars have remained resistant for five years or more, which is about the agronomic lifespan of a cultivar where an active breeding programme exists. However, some cultivars have rusted before they were grown on more than a fraction of the cultivated acreage. In most, if not all the cases, the failures have been due to inadequate knowledge of the virulences present in the pathogen population. In other cases, mutations or perhaps a recombination of existing virulence combinations occurred and rendered the host susceptible. In some instances, the disease screening protocol is inadequate to identify and select the resistant wheat lines.
The failure of resistance over the short term has led to a boom-and-bust syndrome (Kil-patrick, 1975). However, among the breeding programmes for rust resistance, some have been successful for a number of years. The greatest successes have been against stem rust, perhaps because of the nature of the pathogen and perhaps due to the greater number of scientific years of study and work. Green and Campbell (1979) have summarized the success of the Canadian stem rust programme. In Australia, a series of cultivars with Sr26 have been released since 1971 and are now grown on nearly 1 million ha without stem rust losses (Luig and Rajaram, 1972). The adult plant resistance gene Sr2 derived from Hope results in an absence of uredinia in the internode tissues (Hare and McIntosh, 1979; Sunderwirth and Roelfs, 1980). This has been probably the most commonly used Sr resistance gene worldwide since the 1940s. The 1BL.1RS wheat-rye translocation is associated with Sr31, Lr26 and Yr9. Gene Sr31 provides a highly to moderately effective resistance to stem rust and was effective worldwide until 1999. Currently, it is common in many high-yielding wheats, including Aurora, Kavkaz, Burgus II, Lovrin 10, Riebesel, Siouxland, Alondra, Weique, Salzmuendu Bartweizen, Nautica, Clement, Pak 81, Faisalabad 85 and the Veery and Bobwhite crosses from the International Maize and Wheat Improvement Center (CIMMYT).
Although fifty resistance genes have been catalogued for resistance to leaf rust (McIntosh et al., 1998), virulence occurs for a majority of them. Therefore, the best control strategy will involve combinations of these race-specific genes. Several spring wheat cultivars developed by CIMMYT have shown a slow rusting type of resistance (Rajaram et al., 1996). Gene Lr34 together with other un-named slow rusting genes is believed to be involved in the durable resistance of Frontana and other wheats (Singh and Rajaram, 1992). Slow rusting resistance of Pavon 76 involves Lr46 in combination with an unnamed slow rusting gene (Singh et al., 1998).
Biffen (1905) did the first resistance studies for wheat stripe rust. For several reasons, less is known about the resistance to this disease than the other wheat rust diseases. Stripe rust requires somewhat more specialized controls in the greenhouse due to its sensitivity to environmental conditions. Many of the resistances against stripe rust have been of the additive temperature sensitive and/or adult plant types (Lewellen et al., 1967; Robbelen and Sharp, 1978; Sharp and Volin, 1970; Singh and Rajaram, 1994; Wallwork and Johnson, 1984). Some of these resistances are considered non-specific. In Europe, the most durable resistance has been that of Capelle-Desprez (Yr3a, Yr4a, Yr6) (Johnson, 1981), Juliana (Yr14, +), Carstens VI (Yr12, +) and Arminda (Yr13, +) (Stubbs, 1985). In the United States, the cultivars Gaines and Nugaines have provided resistance on a long-term scale (Line et al., 1983). Some CIMMYT germplasm derived cultivars, such as Anza (Yr18) and Pavon 76, also have durable resistance (Singh and Rajaram, 1994). Slow rusting gene Yr18, in combination with other unnamed slow rusting genes, is currently believed to be involved in durable resistance of several spring and winter wheats (Singh, 1992; McIntosh, 1992).
The genes for resistance have been obtained primarily from cultivars of T. aestivum, but some are from other Triticum spp. as well as from Triticum (Aegilops), Secale (rye) and Agropyron. The usefulness or durability of resistance does not seem to be associated with the donor genera or species.
Chemical (fungicide) control
Chemical control has been successfully used in Europe, permitting high yields (6 to 7 tonnes/ha) and where prices for wheat are supported (Buchenauer, 1982; Stubbs and de Bruin, 1970). Chemicals were also used to control a leaf rust epidemic in 1977 in the irrigated Yaqui and Mayo Valleys of Mexico (Dubin and Torres, 1981). Elsewhere, chemicals have had limited use on high-yielding wheats in the Pacific Northwest of the United States for stripe and leaf rust control. Chemical control of leaf rust in the eastern and southern United States has been practised when expected yields exceed 2 tonnes/ha. In Brazil and Paraguay, chemicals are used on wheat with expected yields of 1 tonne/ha and above to control an array of other diseases.
Cultural methods
Cultural practices provide another method for at least partial control of wheat rust epidemics. No single practice is effective under all conditions, but using a series of cultural practices greatly enhances the existing resistances. Farrer’s development and use of early maturing cultivars marked initial successes in controlling stem rust in Australia (McIntosh, 1976). Mexican farmers had learned to sow early to avoid stem rust prior to the use of resistant cultivars (Borlaug, 1954).
Zadoks and Bouwman (1985) emphasized the importance of the green-bridge in carrying the disease from one crop to the next. The green-bridge can be lengthened when some growers plant early and others late. Removing the green-bridge with tillage or herbicides is an effective control measure for epidemics that would result from endogenous inoculum. In some areas, volunteer plants must be controlled several times during the season when wheat is not grown.
Some of the benefits of gene deployment can be obtained by a grower if more than one cultivar is used that differs in resistance and from those grown by immediate neighbours. In some areas, control of timing, frequency and amount of irrigation and fertilization applications can aid in disease control. On large farms, it may help if fields are arranged so that the early maturing cultivars are downwind from late maturing cultivars. Late planting may avoid autumn infections, but late planting may increase the chance of spring infection by exogenous inoculum. As a disease control measure, autumn- and spring-sown wheats probably should not be grown in the same area. Whatever the situation, each cultural practice must be tested against the anticipated types of epidemic that occur in the area.
Eradication of the alternate host
An alternate host eradication programme for stem rust was successful in northern Europe (Hermansen, 1968) and the north-central states of the United States (Roelfs, 1978). Except for Eastern Europe and the northwestern United States, no other areas of the world are known where alternate hosts play any role in stem rust epidemiology. Eradication efforts by individual growers probably would not result in visible gains immediately in stem rust control due to large amounts of asexual inoculum. The alternate host for leaf rust may function more as a source of sexual reproduction than a source of epidemic-generating inoculum. For southern Europe, eradication of Thalictrum or Anchusa would probably not be feasible.
REFERENCES
Anikster, Y., Bushnell, W.R., Eilam, T., Manisterski, J. & Roelfs, A.P. 1997. Puccinia recondita causing leaf rust on cultivated wheats, wild wheats, and rye. Can. J. Bot., 75: 2082-2096.
Azbukina, Z. 1980. Economical importance of aecial hosts of rust fungi of cereals in the Soviet Far East. In Proc. 5th European and Mediterranean Cereal Rusts Conf., p.199-201. Bari, Rome.
Beresford, R.M. 1982. Stripe rust (Puccinia striiformis), a new disease of wheat in New Zealand. Cer. Rusts Bull., 10: 35-41.
Biffen, R.H. 1905. Mendel’s laws of inheritance and wheat breeding. J. Agric. Sci., 1: 4-48.
Borlaug, N.E. 1954. Mexican wheat production and its role in the epidemiology of stem rust in North America. Phytopathology, 44: 398-404.
Buchenauer, H. 1982. Chemical and biological control of cereal rust. In K.J. Scott & A.K. Chakravorty, eds. The rust fungi, p. 247-279. London, Academic Press.
Burdon, J.J., Marshall, D.R. & Luig, N.H. 1981. Isozyme analysis indicates that a virulent cereal rust pathogen is somatic hybrid. Nature, 293: 565-566.
Casulli, F. 1988. Overseasoning of wheat leaf rust in southern Italy. In Proc. 7th European and Mediterranean Cereal Rusts Conf., 5-9 Sept., p. 166-168. Vienna.
Chester, K.S. 1946. The nature and prevention of the cereal rusts as exemplified in the leaf rust of wheat. In Chronica botanica. Walthan, MA, USA. 269 pp.
Craigie, J.H. 1927. Experiments on sex in rust fungi. Nature, 120: 116-117.
Cummins, G.B. & Caldwell, R.M. 1956. The validity of binomials in the leaf rust fungus complex of cereals and grasses. Phytopathology, 46: 81-82.
Cummins, G.B. & Stevenson, J.A. 1956. A check list of North American rust fungi (Uredinales). Plant Dis. Rep. Suppl., 240: 109-193.
d’Oliveira, B. & Samborski, D.J. 1966. Aecial stage of Puccinia recondita on Ranunculaceae and Boraginaceae in Portugal. In Proc. Cereal Rusts Conf., Cambridge, p 133-150.
DeBary, A. 1866. Neue Untersuchungen uber die Uredineen insbesondere die Entwicklung der Puccinia graminis und den Zusammenhang duselben mit Aecidium berberis. In Monatsber Preuss. Akad. Wiss., Berlin, p. 15-20.
de Candolle, A. 1815. Uredo rouille des cereales. In Flora francaise, famille des champignons, p. 83.
Dubin, H.J. & Stubbs, R.W. 1986. Epidemic spread of barley stripe rust in South America. Plant Dis., 70: 141-144.
Dubin, H.J. & Torres, E. 1981. Causes and consequences of the 1976-1977 wheat leaf rust epidemic in northwest Mexico. Ann. Rev. Phytopath., 19: 41-49.
Eriksson, J. 1894. Uber die Spezialisierung des Parasitismus bei dem Getreiderostpilzen. Ber. Deut. Bot. Ges., 12: 292-331.
Eriksson, J. & Henning, E. 1894. Die Hauptresultate einer neuen Untersuchung uber die Getreideroste. Z. Pflanzenkr., 4: 66-73, 140-142, 197-203, 257-262.
Eriksson, J. & Henning, E. 1896. Die Getreideroste. Ihre Geschichte und Natur sowie Massregein gegen dieselben, p. 463. Stockholm, P.A. Norstedt and Soner.
Ezzahiri, B., Diouri, S. & Roelfs, A.P. 1992. The-Role of the alternate host, Anchusa italica, in the epidemiology of Puccinia recondita f. sp. tritici on durum wheats in Morocco. In F.J. Zeller & G. Fischbeck, eds. Proc. 8th European and Mediterranean Cereal Rusts and Mildews Conf., 1992, p. 69-70. Heft 24 Weihenstephan, Germany.
Fontana, F. 1932. Observations on the rust of grain. P.P. Pirone, transl. Classics No. 2. Washington, DC, Amer. Phytopathol. Society. (Originally published in 1767).
Green, G.J. & Campbell, A.B. 1979. Wheat cultivars resistant to Puccinia graminis tritici in western Canada: their development, performance, and economic value. Can. J. Plant Pathol., 1: 3-11.
Groth, J.V. & Roelfs, A.P. 1982. The effect of sexual and asexual reproduction on race abundance in cereal rust fungus populations. Phytopathology, 72: 1503-1507.
Hare, R.A. & McIntosh, R.A. 1979. Genetic and cytogenetic studies of the durable adult plant resistance in ‘Hope’ and related cultivars to wheat rusts. Z. Pflanzenzüchtg, 83: 350-367.
Hart, H. & Becker, H. 1939. Beitrage zur Frage des Zwischenwirts fur Puccinia glumarum Z. Pflanzenkr. (Pflanzenpathol.) Pflanzenschutz, 49: 559-566.
Hendrix, J.W., Burleigh, J.R. & Tu, J.C. 1965. Oversummering of stripe rust at high elevations in the Pacific Northwest-1963. Plant Dis. Rep., 49: 275-278.
Hermansen, J.E. 1968. Studies on the spread and survival of cereal rust and mildew diseases in Denmark. Contrib. No. 87. Copenhagen, Department of Plant Pathology, Royal Vet. Agriculture College. 206 pp.
Hirst, J.M. & Hurst, G.W. 1967. Long-distance spore transport. In P.H. Gregory & J.L. Monteith, eds. Airborne microbes, p. 307-344. Cambridge University Press.
Hogg, W.H., Hounam, C.E., Mallik, A.K. & Zadoks, J.C. 1969. Meteorological factors affecting the epidemiology of wheat rusts. WMO Tech. Note No. 99. 143 pp.
Huerta-Espino, J. & Roelfs, A.P. 1989. Physiological specialization on leaf rust on durum wheat. Phytopathology, 79: 1218 (abstr.).
Hylander, N., Jorstad, I. & Nannfeldt, J.A. 1953. Enumeratio uredionearum Scandinavicarum. Opera Bot., 1: 1-102.
Johnson, R. 1981. Durable disease resistance. In J.F. Jenkyn & R.T. Plumb, eds. Strategies for control of cereal diseases, p. 55-63. Oxford, UK, Blackwell.
Johnson, T. 1949. Intervarietal crosses in Puccinia graminis. Can. J. Res. Sect. C, 27: 45-63.
Joshi, L.M. & Palmer, L.T. 1973. Epidemiology of stem, leaf, and stripe rusts of wheat in northern India. Plant Dis. Rep., 57: 8-12.
Katsuya, K. & Green, G.J. 1967. Reproductive potentials of races 15B and 56 of wheat stem rust. Can. J. Bot., 45: 1077-1091.
Kilpatrick, R.A. 1975. New wheat cultivars and longevity of rust resistance, 1971-1975. USDA, Agric. Res. Serv. Northeast Reg (Rep.), ARS-NE 64. 20 pp.
Le Roux, J. & Rijkenberg, F.H.J. 1987. Pathotypes of Puccinia graminis f. sp. tritici with increased virulence for Sr24. Plant Dis., 71: 1115-1119.
Lewellen, R.T., Sharp, E.L. & Hehn, E.R. 1967. Major and minor genes in wheat for resistance to Puccinia striiformis and their response to temperature changes. Can. J. Bot., 45: 2155-2172.
Line, R.F. 1976. Factors contributing to an epidemic of stripe rust on wheat in the Sacramento Valley of California in 1974. Plant Dis. Rep., 60: 312-316.
Line, R.F., Qayoum, A. & Milus, E. 1983. Durable resistance to stripe rust of wheat. In Proc. 4th Int. Cong. Plant Pathol., p. 204. Melbourne.
Luig, N.H. 1985. Epidemiology in Australia and New Zealand. In A.P. Roelfs & W.R. Bushnell, eds. Cereal rusts, vol. 2, Diseases, distribution, epidemiology, and control, p. 301-328. Orlando, FL, USA, Academic Press.
Luig, N.H. & Rajaram, S. 1972. The effect of temperature and genetic background on host gene expression and interaction to Puccinia graminis tritici. Phytopathology, 62: 1171-1174.
Luig, N.H. & Watson, I.A. 1972. The role of wild and cultivated grasses in the hybridization of formae speciales of Puccinia graminis. Aust. J. Biol. Sci., 25: 335-342.
Maddison, A.C. & Manners, J.G. 1972. Sunlight and viability of cereal rust urediospores. Trans. Br. Mycol. Soc., 59: 429-443.
Mains, E.B. 1932. Host specialization in the leaf rust of grasses Puccinia rubigovera. Mich. Acad. Sci., 17: 289-394.
Mains, E.B. 1933. Studies concerning heteroecious rust. Mycologia, 25: 407-417.
Mains, E.B. & Jackson, H.S. 1926. Physiologic specialization in the leaf rust of wheat, Puccinia triticina Erikss. Phytopathology, 16: 89-120.
Manners, J.G. 1960. Puccinia striiformis Westend. var. dactylidis var. nov. Trans. Br. Mycol. Soc., 43: 65-68.
McIntosh, R.A. 1976. Genetics of wheat and wheat rusts since Farrer: Farrer memorial oration 1976. J. Aust. Inst. Agric. Sci., 42: 203-216.
McIntosh, R.A. 1992. Close genetic linkage of genes conferring adult-plant resistance to leaf rust and stripe rust in wheat. Plant Pathol., 41: 523-527.
McIntosh, R.A., Luig, N.H., Milne, D.L. & Cusick, J. 1983. Vulnerability of triticales to wheat stem rust. Can. J. Plant Pathol., 5: 61-69.
McIntosh, R.A., Hart, G.E., Devos, K.M., Gale, M.D. & Rogers, W.J. 1998. Catalogue of gene symbols for wheat. In Proc. 9th Int. Wheat Genetics Symp., 2-7 Aug. 1998. Saskatoon Saskatchewan, Canada.
Melander, L.W. & Craigie, J.H. 1927. Nature of resistance of Berberis spp. to Puccinia graminis. Phytopathology, 17: 95-114.
Mont, R.M. 1970. Studies of nonspecific resistance to stem rust in spring wheat. M.Sc. thesis. St Paul, MN, USA, University of Minnesota. 61 pp.
Nagarajan, S. & Joshi, L.M. 1985. Epidemiology in the Indian subcontinent. In A.P. Roelfs & W.R. Bushnell, eds. The cereal rusts, vol. 2, Diseases, distribution, epidemiology, and control, p. 371-402. Orlando, FL, USA, Academic Press.
O’Brien, L., Brown, J.S., Young, R.M. & Pascoe, T. 1980. Occurrence and distribution of wheat stripe rust in Victoria and susceptibility of commercial wheat cultivars. Aust. Plant Pathol. Soc. Newsl., 9: 14.
Perez, B. & Roelfs, A.P. 1989. Resistance to wheat leaf rust of land cultivars and their derivatives. Phytopathology, 79: 1183 (abstr.).
Pretorius, Z.A., Singh, R.P., Wagoire, W.W. & Payne, T.S. 2000. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Phytopathology, 84: 203.
Rajaram, S., Singh, R.P. & van Ginkel, M. 1996. Approaches to breed wheat for wide adaptation, rust resistance and drought. In R.A. Richards, C.W. Wrigley, H.M. Rawson, G.J. Rebetzke, J.L. Davidson & R.I.S. Brettell, eds. Proc. 8th Assembly Wheat Breeding Society of Australia, p. 2-30. Canberra, The Australian National University.
Rapilly, F. 1979. Yellow rust epidemiology. Ann. Rev. Phytopathol., 17: 5973.
Robbelen, G. & Sharp, E.L. 1978. Mode of inheritance, interaction, and application of genes conditioning resistance to yellow rust. Fortschr. Pflanzenzucht. Beih. Z. Pflanzenzucht., 9: 1-88.
Roelfs, A.P. 1978. Estimated losses caused by rust in small grain cereals in the United States 1918-1976. Misc. Publ. USDA, 1363: 1-85.
Roelfs, A.P. 1982. Effects of barberry eradication on stem rust in the United States. Plant Dis., 66: 177-181.
Roelfs, A.P. 1985a. Epidemiology in North America. In A.P. Roelfs & W.R. Bushnell, eds. The cereal rusts, vol. 2, Diseases, distribution, epidemiology, and control, p. 403-434. Orlando, FL, USA, Academic Press.
Roelfs, A.P. 1985b. Wheat and rye stem rust. In A.P. Roelfs & W.R. Bushnell, eds. The cereal rusts, vol. 2, Diseases, distribution, epidemiology, and control, p. 3-37. Orlando, FL, USA, Academic Press.
Roelfs, A.P. 1986. Development and impact of regional cereal rust epidemics. In K.J. Leonard & W.E. Fry, eds. Plant disease epidemiology, vol. 1, p. 129-150. New York, NY, USA, MacMillan.
Roelfs, A.P. 1988. Genetic control of phenotypes in wheat stem rust. Ann. Rev. Phytopathol., 26: 351-367.
Roelfs, A.P. & Groth, J.V. 1980. A comparison of virulence phenotypes in wheat stem rust populations reproducing sexually and asexually. Phytopathology, 70: 855-862.
Roelfs, A.P. & Groth, J.V. 1988. Puccinia graminis f. sp. tritici, black stem rust of Triticum spp. In G.S. Sidhu, ed. Advances in plant pathology, vol. 6, Genetics of pathogenic fungi, p. 345-361. London, Academic Press.
Roelfs, A.P. & Long, D.L. 1987. Puccinia graminis development in North America during 1986. Plant Dis., 71: 1089-1093.
Roelfs, A.P. & Martell, L.B. 1984. Ure-dospore dispersal from a point source within a wheat canopy. Phytopathology, 74: 1262-1267.
Roelfs, A.P., Huerta-Espino, J. & Marshall, D. 1992. Barley stripe rust in Texas. Plant Dis., 76: 538.
Rowell, J.B. 1981. Relation of postpenetration events in Idaed 59 wheat seedling to low receptivity to infection by Puccinia graminis f. sp. tritici. Phytopathology, 71: 732-736.
Rowell, J.B. 1982. Control of wheat stem rust by low receptivity to infection conditioned by a single dominant gene. Phytopathology, 72: 297-299.
Rowell, J.B. 1984. Controlled infection by Puccinia graminis f. sp. tritici under artificial conditions. In. A.P. Roelfs & W.R. Bushnell, eds. The cereal rusts, vol. 1, Origins, specificity, structure, and physiology, p. 291-332. Orlando, FL, USA, Academic Press.
Saari, E.E. & Prescott, J.M. 1985. World distribution in relation to economic losses. In A.P. Roelfs & W.R. Bushnell, eds. The cereal rusts, vol. 2, Diseases, distribution, epidemiology, and control, p. 259-298. Orlando, FL, USA, Academic Press.
Saari, E.E., Young, H.C., Jr. & Fernkamp, M.F. 1968. Infection of North American Thalictrum spp. with Puccinia recondita f. sp. tritici. Phytopathology, 58: 939-943.
Samborski, D.J. 1985. Wheat leaf rust. In A.P. Roelfs and W.R. Bushnell, eds. The cereal rusts, vol. 2, Diseases, distribution, epidemiology, and control, p. 39-59. Orlando, FL, USA, Academic Press.
Savile, D.B.O. 1984. Taxonomy of the cereal rust fungi. In W.R. Bushnell & A.P. Roelfs, eds. The cereal rusts, vol. 1, Origins, specificity, structures, and physiology, p. 79-112. Orlando, FL, USA, Academic Press.
Sharp, E.L. & Volin, R.B. 1970. Additive genes in wheat conditioning resistance to stripe rust. Phytopathology, 601: 1146-1147.
Singh, R.P. 1992. Genetic association of leaf rust resistance gene Lr34 with adult plant resistance to stripe rust in bread wheat. Phytopathology, 82: 835-838.
Singh, R.P. & Rajaram, S. 1992. Genetics of adult-plant resistance to leaf rust in "Frontana" and three CIMMYT wheats. Genome, 35: 24-31.
Singh, R.P. & Rajaram, S. 1994. Genetics of adult plant resistance to stripe rust in ten spring bread wheats. Euphytica, 72: 1-7.
Singh, R.P., Mujeeb-Kazi, A. & Huerta-Espino, J. 1998. Lr46: A gene conferring slow-rusting resistance to leaf rust in wheat. Phytopathology, 88: 890-894.
Skovmand, B., Fox, P.N. & Villareal, R.L. 1984. Triticale in commercial agriculture: progress and promise. Adv. Agron., 37: 1-45.
Spehar, V. 1975. Epidemiology of wheat rust in Europe. In Proc. 2nd Int. Winter Wheat Conf., 9-19 Jun., p. 435-440. Zagreb, Yugoslavia.
Stakman, E.C. 1923. Wheat diseases. 14. The wheat rust problem in the United States. In Proc. 1st Pan Pac. Sci. Congr., p. 88-96.
Straib, W. 1937. Untersuchungen uben dasn Vorkommen physiologischen Rassen der Gelbrostes (Puccinia glumarum) in den Jahren 1935-1936 und uber die Agressivitat eninger neuer Formes auf Getreide un Grasern. Arb. Biol. Reichsant. Land. Fortw. Berlin-Dahlem, 22: 91-119.
Stubbs, R.W. 1985. Stripe rust. In A.P. Roelfs & W.R. Bushnell, eds. The cereal rusts, vol. 2, Diseases, distribution, epidemiology, and control, p. 61-101. Orlando, FL, USA, Academic Press.
Stubbs, R.W. & de Bruin, T. 1970. Bestrijding van gele roest met het systemische fungicide oxycaroxin ‘Plantvax’. Gewasbescherming, 1: 99-104.
Stubbs, R.W., Prescott, J.M., Saari, E.E. & Dubin, H.J. 1986. Cereal disease methodology manual, p. 46. Mexico, DF, CIMMYT.
Sunderwirth, S.D. & Roelfs, A.P. 1980. Greenhouse evaluation of the adult plant resistance of Sr2 to wheat stem rust. Phytopathology, 70: 634-637.
Tollenaar, H. & Houston, B.R. 1967. A study on the epidemiology of stripe rust, Puccinia striiformis West in California. Can. J. Bot., 45: 291-307.
Tozzetti, G.T. 1952. V. Alimurgia: True nature, causes and sad effects of the rusts, the bunts, the smuts, and other maladies of wheat and oats in the field. In L.R. Tehon, transl. Phytopathological Classics No. 9, p. 139. St Paul, MN, USA, American Phytopathol. Society. (Originally published 1767).
Tranzschel, W. 1934. Promezutocnye chozjaeva rzavwiny chlebov i ich der USSR. (The alternate hosts of cereal rust fungi and their distribution in the USSR). In Bull. Plant Prot., Ser. 2, p. 4-40. (In Russian with German summary).
Viennot-Bourgin, G. 1934. La rouille jaune der graminees. Ann. Ec. Natl. Agric. Grignon Ser., 3, 2: 129-217.
Wallwork, H. & Johnson, R. 1984. Transgressive separation for resistance to yellow rust in wheat. Euphytica, 33: 123-132.
Watson, I.A. & de Sousa, C.N.A. 1983. Long distance transport of spores of Puccinia graminis tritici in the Southern Hemisphere. In Proc. Linn. Soc. N.S.W., 106: 311-321.
Wright, R.G. & Lennard, J.H. 1980. Origin of a new race of Puccinia striiformis. Trans. Br. Mycol. Soc., 74: 283-287.
Zadoks, J.C. 1961. Yellow rust on wheat studies of epidemiology and physiologic specialization. Neth. J. Plant Pathol., 67: 69-256.
Zadoks, J.C. & Bouwman, J.J. 1985. Epidemiology in Europe. In A.P. Roelfs & W.R. Bushnell, eds. The cereal rusts, vol. 2, Diseases, distribution, epidemiology, and control, p. 329-369. Orlando, FL, USA, Academic Press.